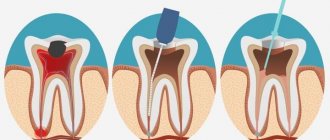
What does granulation tissue look like?
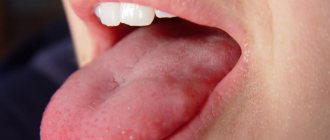
Granulation tissue is young connective tissue.
It develops during the healing of a wound, ulcer, or during encapsulation of a foreign body. Healthy, normal granulation tissue has a pink-red color, granular structure and dense consistency. A cloudy grayish-white purulent exudate is separated from it in small quantities.
Such tissue appears at the borders between dead and living, after injury on the 3-4th day. Granulation tissue consists of many granules that are closely pressed together. They include: amphora substances, loop-shaped vascular capillaries, histiocytes, fibroblasts, polyblasts, lymphocytes, multinucleated vagal cells, argyrophilic fibers and segmented leukocytes, collagen fibers.
Scarring
All wounds on the skin of an adult heal with the formation of a scar, which is a dense formation consisting of connective tissue. What the final scar will look like depends on the degree and nature of the damage, the individual characteristics of the body and other factors.
Sometimes the healing process is accompanied by excessive proliferation of fibroblasts with the formation of a hypertrophic scar limited to the wound area. Even more intense proliferation of fibroblasts can lead to the formation of a keloid. Unlike hypertrophic scars, keloid scars protrude beyond the boundaries of the injured area.
Hypertrophic scarring occurs in all races, although to a lesser extent in young and elderly people. Keloid scarring is more common in people with non-white skin color.
On the other hand, insufficient proliferation of fibroblasts can lead to the formation of an atrophic scar, which is formed due to a lack of collagen. As a result, the scar layer is very thin and the scar area is located below the level of the surrounding skin. This type of scarring is more common in fair-skinned people
Causes of erosion
One of the versions that substantiates the appearance of an erosive process in the stomach is the vital activity of infectious bacteria Helicobacter pylori. This version is confirmed by the detection of antibodies to infection in the blood of over 90% of the examined patients.
Risk factors for the development of erosive gastritis are:
- intoxication and burns on the surface of the stomach;
- surgical manipulations on the digestive tract and heart, organ transplantation and any type of abdominal surgery;
- long-term depression with stress, states of shock and nervous shock;
- gastropathy due to the use of NSAIDs, antiarrhythmic drugs, analgesics, corticosteroids;
- eating poorly chewed, rough, spicy, hot food;
- smoking and alcohol abuse;
- throwing bile into the gastric sac;
- a high increase in the acidity level of gastric juice with a simultaneous decrease in the resistance of the membrane.
In some situations, the appearance of erosive damage to the stomach with the simultaneous presence of benign and malignant neoplasms, inflammatory pathologies of the stomach, lungs and liver, and pathologies of the cardiovascular system is noted. Thrombosis or stagnation of blood in the portal vein is often combined with erosive gastritis. Hemorrhagic erosions are often a complication of a hiatal hernia, concentrated near it.
Return to contents
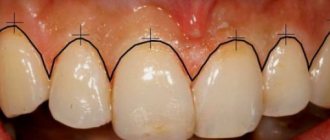
Granulation tissue formation
After just two days, in areas free of blood clots and necrotic tissue, you can notice pink-red nodules - the size of millet granules. On the third day, the number of granules increases significantly and already on the 4-5th day the surface of the wound is covered with young granulation tissue. This process is clearly noticeable on an incised wound.
Healthy, strong granulations are pinkish-red in color, they do not bleed, have a uniform granular appearance, a very dense consistency, and secrete a small amount of purulent, cloudy exudate. It contains a large number of dead cellular elements of local tissue, purulent bodies, admixtures of red blood cells, segmented leukocytes, one or another microflora with the products of its own vital activity.
Due to the fact that in a gaping wound it is impossible for newly formed capillaries to connect with the capillaries of the opposite side of the wound, they bend and form loops. Each of these loops is a framework for the above cells. Each new granule is formed from them. Every day the wound is filled with more and more granules, so the entire cavity is completely contracted.
Types of erosive processes
The erosive process on the gastric mucosa is divided into the following stages:
- Acute erosion - the site of its localization is chosen by the bottom or body of the stomach. It is distinguished by the absence of an epithelial layer, weak infiltration by lymphocytes, and an insignificant fibrin sediment at the bottom.
- Chronic pathology in many situations is noted as erosion of the antrum of the stomach. It is represented by granulation tissue. The bottom of the ulcer is occupied by dilated capillaries with degeneration of the glands, and on the sides there is a layer of hyperplastic epithelium.
Based on the number of formations, the erosive process is divided into:
- single (no more than 1-3 formations located in separate areas of the stomach);
- multiple (more than three in a department).
In accordance with pathomorphological signs, erosion occurs:
- Hemorrhagic. It happens both on the surface and deep. The top is covered with a coating of blood, and next to it there is a pale-colored membrane of edema.
- Flat (or superficial). Erosion with a clear bottom or covered with a light coating, small sides, hyperemic mucosa around the defect like a rim edema.
- Hyperplastic inflammatory (complete erosions). They resemble polyps in appearance, inhabit the top of the gastric folds, and have moderate swelling.
Return to contents
Sequence of events during wound healing
After tissue damage, the initial reaction is usually bleeding. The cascade of vasoconstriction and coagulation begins with thickening of blood that immediately permeates the wound, resulting in hemostasis. Further, as a result of the dehydration process, a scab is formed. This is followed by an influx of inflammatory cells with the release of cellular substances and mediators. Angiogenesis, reepithelialization, and the formation of new cellular and extracellular components occur.
Hemostasis
Initially, the injury causes a drainage of blood and lymph fluid. This process also creates the initial reparative coagulum. In this case, internal and external blood clotting mechanisms are involved. The internal mechanism is carried out with the help of platelets, and the external mechanism occurs with the direct participation of tissue factors.
We suggest you read how long after tooth extraction can the next tooth be removed
Following vasoconstriction, platelets adhere to the damaged endothelium and release adenosine diphosphate (ADP), promoting platelet aggregation and wound closure. Once the short-term vasoconstriction is complete, the vessels dilate, allowing more platelets and other blood cells to flow in.
At this stage we can talk about the beginning of the inflammatory phase. Sometimes the inflammatory phase is isolated as a separate phase, although it begins during the hemostasis phase, confirming the overlapping nature of the healing processes. Platelets and white blood cells secrete many factors that speed up the healing process.
Alpha granules release platelet-derived growth factor (PDGF), platelet-derived factor IV and transforming growth factor (TGF)-β). The processes of inflammation, collagen degradation and collagenogenesis, the formation of myoblasts from transformed fibroblasts, the growth of new blood vessels and re-epithelialization occur.
These processes are mediated by a variety of cytokines and growth factors. Interleukins strongly influence the inflammatory process. Vascular endothelial growth factor (VEGF) and other factors accelerate the formation of blood vessels, and some of them have multiple functions, such as fibroblast growth factor (FGF)-2, which affects not only the process of angiogenesis but also the process of re-epithelialization.
Vasoactive amines such as histamine and serotonin are released from dense bodies found in platelets. Platelet-derived growth factor (PDGF), along with Transforming growth factor beta (TGF-β), is a potent modulator of fibroblastic mitosis. Mitosis causes the formation of fertile collagen fibrils in later stages.
Inflammation
The inflammatory phase begins during the hemostasis phase. Early in the inflammatory phase, polymorphonuclear leukocytes (PMN) predominate, while in the later phase, monocytes/macrophages predominate.
During the first 6-8 hours, the next phase of the healing process begins, the amount of PMN in the wound increases. TGF-β facilitates the migration of PMNs from surrounding blood vessels. These cells cleanse the wound. PMNs reach their maximum amount after 24-48 hours. By about 72 hours their concentration begins to decrease.
As the inflammation process further develops, monocytes are released from the vessels. After monocytes leave the vessels, they are called macrophages. Macrophages continue the cleansing process and produce various growth factors within 3-4 days. Macrophages control the proliferation of endothelial cells with the sprouting of new blood vessels and duplication of smooth muscle cells.
Granulation
This stage consists of several subphases. These subphases do not occur in discrete time frames, but represent a general and continuous process. The subphases are fibroplasia, matrix deposition, angiogenesis and reepithelialization.
Clinical symptoms of erosive stomach
The location of the erosive process in the stomach varies. And often its clinic is determined by the type and location of pain and other sensations.
For example, erosion of the antrum causes uncomfortable impulses in the navel area, damage to the body of the stomach - pain is felt under the left hypochondrium.
The symptom complex consists of hemorrhagic and ulcer-like symptoms.
Ulcer-like signs are characteristic of different stages of erosive inflammation:
- pain after eating;
- less often - pain when the stomach is empty;
- frequent attacks of heartburn;
- nausea, airy belching.
The presence of hemorrhagic signs is observed in 1/5 of patients with gastric erosion:
Usually the acute stage of erosive gastritis ends with rapid (from 5 to 15 days) epithelization of the affected areas. Upon completion of their healing, no traces are recorded on the gastric mucosa.
Hyperplastic erosions often take a chronic course and exist for several years, after which they disappear. Some erosions of this kind exist for quite a long time, worsening as they are exposed to irritants, and then healing.
Return to contents
Layers
The layers of granulation tissue are divided:
- to superficial leukocyte-necrotic;
- the layer of granulation tissue itself;
- fibrous deep layer.
Over time, the growth of capillaries and cells decreases, and the number of fibers increases. Granulation tissue begins to turn first into fibrous tissue, and then into scar tissue.
The main role of granulation tissue is barrier function; it prevents microbes, toxins, and decay products from entering the wound. It inhibits the vital activity of microbes, dilutes toxins, binds them, and helps to reject necrotic tissue. Granulations fill the defect cavity, wounds, and a tissue scar is created.
Enzyme treatment
Modern research in the field of wound healing is aimed at studying agents that affect the processes associated with the restoration of damaged tissue. Laser techniques and other techniques are used to enhance cell proliferation, cell migration, and accelerate wound healing. Human cell conditioned media have been shown to improve skin healing time after laser.
We suggest you read: When do you need to remove teeth when installing braces and are there any alternatives?
Agents such as platelet-rich plasma (PRP) and erythropoietin (EPO) are modulators that have positive effects on tissue regeneration and have been successfully used to promote wound healing. Nutritional factors are also important for proper wound healing. Improved nutritional status in adults correlates with improved wound healing.
Honey has been shown to be less beneficial for wound healing despite its use since early times. Honey also causes delayed healing in certain types of wounds. On the other hand, there is published evidence that treatment with honey leads to faster healing of shallow burns compared to conventional treatments.
According to some studies, medications with the greatest risk of negatively affecting wound healing and skin integrity include antibiotics, anticonvulsants, angiogenesis inhibitors, steroids, and nonsteroidal anti-inflammatory drugs. On the other hand, medications that promote wound healing include ferrous sulfate, insulin, thyroid hormones and vitamins.
Studies on rats have shown that Turkish pine (Pinus brutia) bark increases the rate of wound healing. It has also been found that aloe arborescens has better healing properties compared to aloe vera. Appropriate neurological stimulation is also important for wound healing.
Stem cells continue to be a new frontier of research in the arsenal of wound healing strategies. Stem cells, particularly adipose stem cells, have been shown to improve wound healing. Further research in this area appears promising. Exogenous stem cells are obtained from mesenchyme, usually obtained from bone marrow but available from other sources, and are used in non-healing inflammatory wounds
Recently, special attention has been paid to the properties of enzymes. This is especially true for proteases. To treat fibrin enzymes, substances of this type are used. They help dissolve excess protein, thereby preventing serious complications such as blood clots.
The properties of proteolytic enzymes are different. They are able to have fibrinolytic and immunomodulatory effects on the body, as well as improve blood circulation and work as decongestants and anti-inflammatory substances.
Since thrombus formation is based on the production of fibrin, a protease is needed that causes the breakdown of this substance. Without such an enzyme, it is impossible to break the protein into molecules, therefore, there will be no improvement in blood microcirculation.
With local exposure to protease, it is possible to remove necrotic plaque, resolve fibrinous formation, and liquefy viscous secretions.
Complications after erosion
Erosion is dangerous because it can cause hidden (asymptomatic) bleeding, which gradually undermines the health of the body as a result of a decrease in hemoglobin in the blood with the development of anemia.
Large hemorrhagic forms of erosion sometimes reveal severe bleeding, manifested by acute conditions (bloody diarrhea or vomiting). Such signs of deteriorating health require an immediate call to the ambulance.
The risk of malignant neoplasm arising from erosion is controversial. Observations indicate that malignant erosions occur extremely rarely. Basically, the fixation of a stomach cancer is evidence of the primacy of the malignant process.
And, conversely, visualized long-term non-epithelializing erosive processes (especially in elderly patients) are the reason for conducting a thorough examination of the colon, liver, pancreas due to the secondary nature of individual ulcers, which accompany the clinic of the main pathology.
Hyperplastic chronic forms of inflammatory erosions can act as a trigger for the appearance of polyps. The latter require surgical removal only.
An unpleasant consequence of gastric erosion is its combination with reflux esophagitis, gastric hernia, atrophic form of gastritis, and the transition of erosion into peptic ulcer.
The final stage of the plasma coagulation cascade consists of the formation of insoluble fibrin from the soluble plasma protein fibrinogen under the influence of thrombin and f.XIII (Fig. 42).
Rice. 42. Consecutive stages of formation of insoluble fibrin
from soluble fibrinogen
Thrombin
Thrombin is a key enzyme in hemostasis. Thrombin, a vitamin K-dependent protein, is a serine protease. The liver synthesizes the inactive precursor prothrombin, which subsequently circulates in the plasma. In the f.Xa-Va-II complex on the phospholipid surface, limited proteolysis of prothrombin occurs. Several active structures with decreasing molecular weight are formed - mesothrombin, α-thrombin ,
β
-
thrombin, γ-thrombin. The most significant product is a serine protease - α-thrombin. On mo-
The thrombin molecule has at least 4 binding sites for substrates, inhibitors, cofactors and calcium ion. This, as well as the ability of thrombin to actively function not only in the solid phase, but also in the bloodstream, allows it to perform numerous functions. The most important functions of thrombin in hemostasis:
Limited proteolysis of fibrinogen to fibrin monomers (occurs in the liquid phase - the bloodstream).
Activation of f.V, -VIII, -VII, -XI.
Platelet activation.
In combination with thrombomodulin, thrombin activates protein C.
Activation of f.XIII.
Limited proteolysis of plasma carboxypeptidase B to its active form, thrombin-activated fibrinolysis inhibitor (TAFI).
Stimulation of the release of tissue plasminogen activator from endothelial cells. However, the role of thrombin in the body is not limited to the above functions. A key role in the process of blood coagulation, activation of the vascular endothelium, cell growth and repair processes, activation of peripheral blood cells, activation of fibrinolysis - these are the most studied functions of thrombin. Apparently, over time this list will increase significantly.
Plasma hemostasis proteins
An indirect confirmation of the importance of thrombin for the body can be the fact that only a few descriptions of patients with a homozygous defect in the thrombin molecule are known, and patients with hypoprothrombinemia are extremely rare.
The most important thrombin inhibitor is antithrombin III. The heparin cofactor P plays a somewhat lesser role.
Factor XIII
Factor XIII is a transglutaminase. In plasma, most of the inactive f.XIII is associated with fibrinogen. Activation of f.XIII occurs through limited proteolysis of inactive f.XIII by thrombin simultaneously with the cleavage of peptide A from fibrinogen. Like most other enzymes, it has several functions in hemostasis:
Stabilizes the fibrin clot by forming covalent bonds between the y-chains of fibrin monomers.
Participates in the binding of the α-inhibitor of plasmin to fibrin, which helps prevent the premature lysis of the fibrin clot.
F.XIII plays a significant role in the processes of polymerization of actin, myosin and other components of the platelet cytoskeleton, which is extremely important for platelet activation and retraction of the formed fibrin clot. This explains the presence of f.XIII in the cytoplasm of platelets.
Cross-reactions of f.XIII with f.V and von Willebrand protein were detected. In addition to direct hemostatic reactions,
f.XIII is involved in the formation of connective tissue and reparative reactions:
Participates in the binding of fibronectin molecules to each other and to fibrin molecules. This is probably important for directed cell migration and repair processes.
Plays a role in collagen biosynthesis, catalyzing the formation of bonds between collagen molecules of types I, II, III and V.
blood and form a strong three-dimensional structure that effectively closes the damage to the vessel and prevents blood loss. The concentration of fibrinogen in the blood of a healthy person is significantly higher than the concentration of other hemostasis proteins, which is due to its unique role.
Fibrinogen synthesis occurs in the liver and is independent of vitamin K. Some fibrinogen is synthesized in megakaryocytes and contained in platelets. This fibrinogen is somewhat different from the fibrinogen synthesized in the liver.
In addition to hepatocytes and megakaryocytes, fibrinogen γ-chain gene activity has been detected in several other tissues - brain, lungs, bone marrow, where fibrinogen γ-chains appear to act as adhesion molecules.
Fibrinogen is a large multicomponent protein that consists of three pairs of polypeptide chains - 2α, 2β, 2γ, interconnected by disulfide bridges and intertwined relative to each other (Fig. 43).
The spatial structure of the fibrinogen molecule consists of a central E-domain and 2 peripheral D-domains. The α- and β-chains form globular structures - fibrin peptides A and B (FPA and FPV), which close the complementary sites in fibrinogen and do not allow this molecule to polymerize. The process of interaction between fibrinogen and thrombin occurs in the liquid phase - the bloodstream. Thrombin combines with fibrinogen and cleaves off the final sequences from the α- and β-chains - 2 FPA and 2 FPV (Fig. 44). Ra-
Fibrinogen.
Formation of a hemostatic thrombus
Fibrinogen is a unique molecule that has the property of quickly polymerizing in a current
Rice. 43. Fibrinogen
consists of 3 paired protein molecules α, β and γ, Fibrinopeptides A and B (FPA and FPV) are cleaved from fibrinogen by thrombin, thereby initiating the polymerization process and the conversion of fibrinogen into fibrin
Plasma hemostasis proteins
Rice. 44. Formation of fibrin monomers
from fibrinogen. Thrombin cleaves the fibrinopeptides FPA and FPV from the fibrinogen molecule, thereby forming soluble fibrin monomers, which are capable of polymerizing to a linear polymer, or “soluble fibrin”
soluble fibrin monomers. Subsequently, spontaneous connection of complementary sections of fibrin monomers occurs. First, dimers are formed, then oligomers, and finally monofilaments of polymerized fibrin are assembled. Thus, the fibrin chain is formed by spontaneous, end-to-end polymerization of fibrin monomers, in which the terminal part of one monomer interacts with the central part of another monomer at the site of FPA cleavage. The result of such polymerization is a linear polymer 2 molecules wide (Fig. 44). At this stage, fibrin is easily soluble in 5 molar
urea, which is why it is called soluble fibrin.
By combining with fibrinogen, thrombin not only cleaves off fibrinopeptides. but also activates the associated factor XIII. Factor XIIIa forms covalent bonds between the γ-chains (D-domains) of soluble fibrin filaments (Fig. 45), which are connected through the formation of peptide bridges between the side radicals of lysine and glutamine. Fibrin monofilaments crosslinked together form a strong network that is less susceptible to fibrinolysis and more resistant to mechanical stress. In this form, fibrin does not dissolve in 5-molar urea and is called insoluble fibrin.
The resulting fibrin clot is a three-dimensional molecular network that includes platelets, erythrocytes and leukocytes (Fig. 46). Activated platelets bound to fibrin threads through GPIIb-IIIa receptors reduce
Rice. 45. Formation of insoluble fibrin
under the influence of factor XIIIa
Plasma hemostasis proteins
under the influence of thrombostenin (platelet actomyosin) due to their inherent contractile properties (see chapter “Platelets”). Retraction of the blood clot occurs. The clot is compacted, and part of the whey is squeezed out of it. The formation of the final thrombus occurs 10-15 minutes after fibrin polymerization.
If platelets are absent or have a GPIIb-IIIa defect, then retraction of the blood clot does not occur and it is quickly lysed through the process of fibrinolysis. In the absence of thrombus retraction in the vascular bed, separation of thrombotic masses and embolism of distant vessels (thromboembolism) is possible.
The transformation of prothrombin into thrombin is carried out by the prothrombinase complex. In this case, the F 1+2 fragment, which is stable in plasma, is cleaved from prothrombin, and its detection by immunological methods is used as one of the markers of global activation of coagulation (thrombinemia). During further proteolysis, the single-chain prothrombin molecule is transformed into meizothrombin, and then into the double-chain active enzyme thrombin (factor IIa).
Thrombin, which directly acts on fibrinogen and leads to the formation of fibrin, is the main effector molecule in the coagulation cascade.
Wound healing
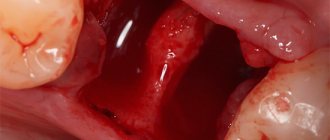
Granulations always form at the boundaries between living and dead tissue. They form faster when there is good blood circulation in the damaged tissue. There are cases when granulations form at different times and develop unevenly. This depends on the amount of dead cells in the tissue and the timing of their rejection.
The faster granulation occurs, the faster the wounds heal. After cleansing the wound of dead tissue and inflammatory exudate, the granulation layer becomes clearly visible. Sometimes in medical practice it is necessary to remove granulation tissue; most often this is used in dentistry during gingivotomy (gum incision).
If there are no reasons preventing healing, the entire wound cavity is filled with granulation tissue. When granulations reach the skin level, they begin to decrease in volume, become a little paler, then become covered with skin epithelium, which grows from the periphery to the center of the damage.
We invite you to read: A child’s baby tooth is not falling out: is it necessary to consult a dentist? Baby teeth have fallen out and new ones are not growing - should we sound the alarm?
The role of fibrin in inflammation
Fibrin networks limit the focus of inflammation
Fibrin synthesis begins immediately after fibrogen comes into contact with the enzyme thrombokinase, which is released from damaged or destroyed tissues. After this, a fixation reaction occurs, during which fibrin captures pathological substances and blocks them. Thus, fibrin prevents inflammation from spreading to healthy tissue.
Fibrin also prevents the inflammation itself from growing. At its very initial stages, when active division of leukocytes and their migration to the site of inflammation has not yet begun, fibrin molecules surround the site in a circle and prevent its spread.
Summary
Research into the processes involved in wound healing dates back to ancient times and continues to this day. Interest grew in the 1900s, and by 1960 it was realized that wound healing time could be reduced by up to 50% if the right conditions were created. Since this time, the number of studies has been systematically increasing.
The wound healing process is a collection of interrelated and related events. Understanding of these processes and the factors that influence them continues to expand.
Healing by primary and secondary intention
Wound healing can occur by primary or secondary intention, depending on its nature.
Primary intention is characterized by a reduction in the edges of the wound due to the connective tissue organization of granulation. It firmly connects the edges of the wound. After initial tension, the scar remains almost invisible and smooth. Such tension can tighten the edges of the wound slightly if the opposite sides are at a distance of no more than one centimeter.
Secondary intention is characteristic of the healing of large wounds, where there is a lot of non-viable tissue. Significant defects or all purulent wounds undergo healing by secondary intention. Differing from the primary type, secondary intention has a cavity, which is filled by granulation tissue.
The scar after secondary intention has a pale red color and protrudes slightly beyond the surface of the skin. As the vessels in it gradually thicken, fibrous and scar tissue develops, keratinization of the skin epithelium occurs, the scar begins to turn pale, becomes denser and narrower. Sometimes scar hypertrophy develops - this is when an excess amount of scar tissue forms.
When does the disease occur?
- Inflammation in the distal parts of the esophagus occurs after scarlet fever, diphtheria. The lesion can be primary, that is, it begins in the esophagus, or secondary, that is, it enters the organ through the bloodstream.
- Fungal infections are a risk factor.
- In adults, this condition can occur after radiation therapy, with blood diseases, and malignant neoplasms.
- The presence of reflux (reflux of gastric contents into the esophagus) additionally injures the mucous membrane of the distal section.
Differences from other forms of esophagitis
Esophagitis combines inflammatory processes of the esophagus, but changes in the organ itself can be different, depending on the cause of the disease, the presence of reflux. In the fibrinous form, films form on the walls of the esophagus; in most cases, they are easily removed, since they are associated only with the mucous membrane. This disease is also called pseudomembranous esophagitis.
In severely ill patients, fibrin plaque grows into the submucosal ball, firmly connecting to the esophagus. After removing the films, erosions and shallow ulcers remain that bleed for a long time. Treatment in this case will be aimed at speedy healing of the defect. The result of reflux in the distal esophagus is catarrhal (superficial) inflammation.
Pathologies of granulation
If the wound process is disrupted, pathological granulations may form. Insufficient or excessive growth of granulation tissue, disintegration of granulations, and premature sclerosis are possible. In all these cases, and also if the granulation tissue bleeds, special treatment will be required.
The development of granulations and epithelization processes fade away if there are such unfavorable factors as deterioration of blood supply, decompensation of any systems and organs, oxygenation, or repeated purulent process. In these cases, granulation pathologies develop.
The clinical picture is as follows: there is no contraction of the wound, the appearance of the granulation tissue changes. The wound looks pale, dull, loses turgor, becomes cyanotic, and becomes covered with a coating of pus and fibrin.
Tuberous granulations are also considered pathological when they protrude beyond the edges of the wound - hypergranulations (hypertrophic). Hanging over the edges of the wound, they interfere with the process of epithelization. In these cases, they are cauterized with concentrated solutions of potassium permanganate or silver nitrate. The wound continues to be treated by stimulating epithelization.
Medicines
If indicated, the patient is prescribed a number of medications. Most often these are analgesics, antihistamines and detoxification compounds. During the treatment of fibrinous plaque, immune stimulants are used. It is necessary to take into account that:
- if there is a threat of infection spreading, surgeons prescribe antibiotics;
- physicians must constantly monitor treatment, monitor the recovery process and adaptation during the postoperative period;
- attention is paid to how severe the inflammatory processes are in the wound itself, and whether it has become covered with plaque again.
It is impossible to do without studying material from the problem area, the patient’s blood. It is important to timely study the microbial spectrum in fibrinous plaque. In this case, even with a severe infection, treatment will be successful and without critical consequences.
Video: fibrin threads under a microscope
Did you find useful information for yourself? Want to read more about this topic? Like ♥, subscribe to our channel and you will be one of the first to know about new publications!
And if you have something to share, leave your comments! Your feedback is very important to us!
Injuries, often with severe damage to the skin and tissues, are fortunately not an everyday situation, but, alas, not excluded.
Inflammation of the wound, the causes of which can be very diverse, is a natural process in severe wounds.
If you do not respond to wound inflammation in time, the symptoms can intensify significantly and enter a critical phase, leading to serious complications. We invite you to familiarize yourself with the main signs that characterize wound inflammation, the treatment of which, if simple rules are followed, can be quick and effective.
The importance of granulation tissue
So, to summarize, let’s highlight the main roles played by granulation tissue:
- Replacement of wound defects. Granulation is a plastic material that fills the wound.
- Protecting the wound from foreign bodies, organisms, and toxins. This is achieved thanks to a large number of leukocytes, macrophages, as well as a dense structure.
- Rejection and sequestration of necrotic tissue. The process is facilitated by the presence of macrophages, leukocytes, as well as proteolytic enzymes that secrete cellular elements.
- During the normal course of healing, epithelialization begins simultaneously with granulation. Granulation tissue is transformed into coarse fibrous tissue, and then a scar is formed.
Symptoms
- The pain intensifies while eating. Food irritates erosive areas, promotes the separation of films and the appearance of new defects. This applies to a greater extent to spicy, hot, coarse dishes.
- Swallowing problems (dysphagia).
- Belching, bad breath, heartburn in the presence of reflux are common complaints of patients.
- Nausea can result in vomiting with blood and the presence of fibrin films. Erosive changes are manifested by hemoptysis.
Diagnostics
For successful treatment, the doctor must conduct a full examination.
- Careful collection of complaints plays a key role. Chest pain while eating, heartburn, vomiting with blood or fibrin films are key signs of pseudomembranous esophagitis. If it is possible to find a connection with scarlet fever or diphtheria, this simplifies diagnosis and treatment.
- A general blood and urine test is necessary to determine the severity and severity of the inflammatory process.
- pH testing can indicate the presence of reflux; this method measures the acidity in the distal esophagus.
- The main diagnostic method is endoscopy. The doctor visually assesses the condition of the mucous membrane, catarrhal or fibrinous inflammation, and the degree of damage. If necessary, a biopsy is taken.