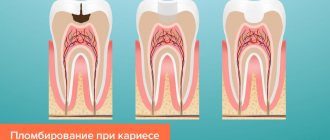
Modern materials for filling teeth
The following groups of dental cements : zinc phosphate (phosphate cement, visfat cement, uniface), bactericidal (phosphate cement with silver, dioxyvisfate), zinc-xide eugenol cements (Kariosan), silicate (silicin, silicin-2, alumodent) , silicophosphate (silidont, lactodont, infantid), polycarboxylate cements, glass ionomer cements. Zinc phosphate cements . Powder and liquid are available as a set. Powder components: 75-90% zinc oxide (providing adhesion), with the addition of silicon oxide (giving glassiness, transparency, shine), magnesium oxide (increasing ductility and mechanical strength), calcium oxide (accelerating setting, viscosity), aluminum oxide (increasing strength and hardness). The liquid is an aqueous solution of orthophosphoric acid. Phosphate cement. The powder is yellow or light yellow in color and consists of 90% zinc oxide, 60/0 magnesium oxide, 4% calcium oxide. Liquid - 35% aqueous solution of orthophosphoric acid, with the addition of phosphates: zinc, aluminum, magnesium to reduce the rate of interaction between powder and liquid.
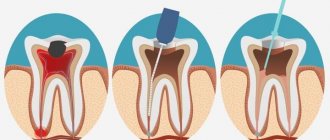
Kneading technique . Powder and liquid are separately applied to the smooth surface of the glass in a ratio of 4:1. The powder is divided into approximately 4 parts, added sequentially to the liquid and thoroughly ground. The mass is considered correctly mixed if it does not reach behind the spatula when it is torn off, but breaks off, forming 1 mm teeth. If the mass turns out to be thick, you cannot add liquid; you need to prepare it again. The maximum adhesive period of the filling test is 4-8 minutes. Positive properties : good adhesion, coefficient of thermal expansion is close to the coefficient of thermal expansion of tooth tissue, does not irritate the pulp, impermeable to acids and monomers of permanent fillings , has low thermal conductivity, radiopaque. Negative properties : absorbs in oral fluid, low mechanical strength, does not have anti-inflammatory and antiseptic effects, does not have aesthetic qualities. Indications for use: as an insulating gasket under a permanent filling ; for permanent filling under an artificial crown or for filling baby teeth; Root canal filling according to indications; fixation of orthopedic structures. Uniface. Unified phosphate cement. Due to the ammonium molybdate-based matrix, it has good adhesion to both metal and tooth tissue, is more durable and chemically resistant. Visphat cement (bismuth cement). The powder has the same composition as phosphate cement, but with the addition of 3% bismuth oxide. Available in 3 shades: light yellow, golden yellow and dark yellow. Liquid - 37% aqueous solution of orthophosphoric acid. It has good adhesion, hardens faster, is more durable and radiopaque, less soluble, has bactericidal and bacteriostatic properties, but is capable of changing the color of hard tooth tissues. The maximum adhesive period of the filling test is 3-3.5 minutes. The mixing technique is similar to mixing phosphate cement. Indications for use : as an insulating gasket under a permanent filling ; for permanent filling under an artificial crown or for filling baby teeth; fixation of orthopedic structures. Bactericidal . Phosphate cement with silver, Argyl, Unitsem. Foscem is a phosphate cement with the addition of silver. Has pronounced bactericidal properties. The preparation method and application are similar to phosphate cement, but it cannot be used as a spacer under a filling on frontal teeth, since silver stains the tooth gray. Dioxyvisfate with the addition of dioxidine. Cement also has bactericidal properties, is mechanically strong, slightly soluble, and does not stain tooth tissue. Silicate. Silitsin, Silitsin-2, Velatsin. Available in powder-liquid kits. Powder components - silicon oxide (29-47%), aluminum oxide (15-35%), calcium (up to 14%), fluorides (up to 15%), a small amount of magnesium, iron, phosphorus salts. Available in 7 colors. Positive properties : the color is close to enamel, fluoride compounds impart anti-cariogenic properties, reduce the solubility of enamel, and reduce the possibility of secondary caries. Disadvantages : toxic, therefore used on living teeth only with an insulating gasket, weak adhesion (no zinc oxide), low mechanical strength (fragility and brittleness), relatively high solubility in the oral cavity. Kneading technique . Apply 1 g of powder and 5-7 drops of liquid to the smooth surface of the glass. The powder is gradually added to the liquid and mixed with a plastic spatula, since the metal changes the color of the filling. Indications for use : filling the frontal group of teeth in the presence of cavities of classes 3, 4, 5 according to Black. Silicophosphate cements are silicate cements modified with zinc phosphate cements. Silidont, Veladont . Available as a powder-liquid kit. Powder - 80% silicine powder and 20% phosphate cement. The liquid is a solution of orthophosphoric acid. Positive properties : good adhesion, mechanical strength and chemical resistance are higher than that of silicine. Negative properties : toxic, not aesthetic (white, opaque), not strong enough and resistant compared to modern filling materials. The mixing technique is similar to silicine. Indications for use : filling the chewing group of teeth, grades 1,2, 5 according to Black. Filling with phosphate, bactericidal, silicate, silicophosphate, and polycarboxylate cements should be carried out under conditions of isolating the tooth from saliva and thoroughly drying the cavity to be filled. Glass ionomer cements. Glass ionomer cement (polyalkene cement) consists of components typical of dental cements - powder and liquid, which harden due to an acid-base reaction. Conventional glass ionomer cements use aqueous solutions of polycarboxylic acids (polymers of alkene acids), for example, polyacrylic acids and their copolymers with itaconic or maleic acid. The carboxyl group of the polymeric acid interacts with calcium ions and a strong polymer matrix is formed. The same acid provides chemical adhesion to calcium of enamel and dentin. Available in chemical, light curing and double curing. Because of freeze-drying, acids can be added directly to the powder, increasing the accuracy of liquid and powder dosing. The liquid component of water-hardening glass ionomer cements consists of distilled water or tartaric acid. The powder component consists of calcium-aluminum-silicate glass containing crystallized droplets saturated with calcium fluoride. Fluoride, after applying the filling, is released into the oral cavity for a long time, providing organic anti-caries protection in the marginal area of the filling. The binding reaction of both main components occurs in 3 stages. The acid releases calcium and aluminum ions from silicate glass. Since calcium ions are released faster, they are the first to react with the acid. After wetting the calcium bridges with polyacrylate acid, a carboxylate gel is formed, which is sensitive to moisture and drying. When moisture initially enters, the binding time increases, strength and hardness decrease, loss of transparency, porosity and roughness of surfaces, and increased erosion of the filling . As glass ionomer cement dries, it becomes opaque, cracks, and does not fully bond. Therefore, protection through varnishes, bonding or matrices is necessary. Aluminum ions penetrate the matrix within a few hours, forming a water-soluble calcium-aluminum-carboxylate gel. The sintering method can be used to fuse metal into glass particles. Used for this purpose in most cases, silver serves as a shock absorber and increases bending strength and abrasion resistance. Glass modified in this way is called cermet cement (ceramics-metal-glass ionomer cement). The third group includes light- and dual-curing glass ionomer cements, the liquid components of which, in addition to acid, contain, for example, hydrophilic monomers (hydroxyl methacrylate - HEMA), Bis-GMA and photoaccelerators. Due to light copolymerization of methacrylate with polyacrylic acid groups, covalent and ionic bonds are formed, which contribute to the hardening of the material. Method of application. Before taking, the powder is thoroughly mixed. Mix in a strict powder-liquid ratio specified by the manufacturer. After taking the powder, you should carefully and tightly close the lid of the bottle, since the powder is hygroscopic. It is necessary to apply cement to a slightly moistened, not overdried surface, since the high concentration of ions promotes the diffusion of dentinal tubule fluid outward. In addition, glass ionomers are hydrophilic, they absorb small amounts of liquid from the surface of dentin and dentinal tubules, which improves adhesion. Therefore, immediately after adding the material, the filling is covered with protective varnishes (they are supplied as a set) to prevent the penetration of liquid from the oral cavity. The final finishing of the filling is carried out after 24 hours. Positive properties : chemical adhesion that does not require acid etching; biological compatibility with dental ; no toxic effect on the pulp; gradually released fluorine enters the tooth tissue and increases the tooth’s resistance to demineralization (anti-carious effect); low polymerization shrinkage; the coefficient of thermal expansion is close to the coefficient of thermal expansion of tooth tissue ; radiopaque. Disadvantages: insufficient mechanical strength; satisfactory aesthetic qualities. Indications for use: filling cavities mainly of classes III and V, filling non-carious lesions of teeth (hypoplasia, wedge-shaped defects, erosions); filling cavities of primary teeth of all classes; as insulating gaskets; as a sealant; creating the basis for the restoration (the main part of the cavity is filled with ionomer cement, and the surface layer is filled with a composite material); restoration of the tooth crown under an artificial crown, inlay; restoration of the crown stump before taking an impression; fixation of orthopedic structures; filling .
These materials are, in most cases, a powder-liquid system. The chemical reaction underlying curing is acid-base. The final product is a substance slightly soluble in water and oral fluid.
Zinc-eugenol cement (ZEC). Despite its solid history, this cement is still used in practice. There are simple and enhanced versions of COE. Simple is used in cases where strength and solubility are not critical parameters. The strengthened version contains aluminum oxide, rosin and polymethyl methacrylate and is characterized by increased strength and less solubility. It is used for temporary fillings, gaskets, etc.
These materials are, in most cases, a powder-liquid system. The chemical reaction underlying curing is acid-base. The final product is a substance slightly soluble in water and oral fluid.
Zinc-eugenol cement (ZEC). Despite its solid history, this cement is still used in practice. There are simple and enhanced versions of COE. Simple is used in cases where strength and solubility are not critical parameters. The strengthened version contains aluminum oxide, rosin and polymethyl methacrylate and is characterized by increased strength and less solubility. It is used for temporary fillings, gaskets, etc.
As is known, eugenol has an antimicrobial, sedative and mild irritant effect, which has a beneficial effect on reparative processes in the pulp. The biocompatibility of this cement is very high. Unfortunately, even the most durable versions of COE cannot be used for permanent filling or cementation. Eugenol may cause hypersensitivity in some patients and skin irritation in staff. It is also necessary to remember the effect of eugenol on the polymerization of composites. Calcium hydroxide can be used to block eugenol; the reaction produces insoluble calcium eugenate. It should be remembered that eugenol easily oxidizes, so it should be stored in small, tightly closed dark glass bottles. The liquid should be transparent, slightly yellowish. A change in color to brown indicates its oxidation and loss of properties.
Paste-to-paste materials are mixed in equal proportions until a uniform color is achieved. Materials of the powder-liquid type are mixed on a glass plate by adding first large portions of powder to the liquid, then smaller ones. Mixing requires some effort to achieve a homogeneous paste-like consistency. To cement structures, mixing is carried out to the point where, when the spatula is torn from the cement, a trace of 1-2 cm stretches behind its flat surface. To form a gasket or temporary filling, a thicker, dough-like consistency is required. At the same time, the material stops sticking to tools and can be processed with a smoothing iron and a plugger.
Cleaning of instruments, as with other cements, must occur before curing. Soap makes it easier to clean up unhardened paste. Hardened paste can be cleaned with alcohol or any other organic solvent.
The hardening of COE is accelerated in the presence of water, so cement hardens faster in the oral cavity than on glass. Excess cement is removed after it has completely cured. It becomes brittle and easily chips along the edges of the crown using a trowel or probe.
Examples of COE include Kalsogen Plus, Dentsply; Cavitec, Kerr; "Zinoment", Voco.
Zinc phosphate cement. It has been used in dentistry for several centuries. Once upon a time it was the strongest and most reliable cement, but even today, despite the emergence of materials with better performance, it is quite popular.
Zinc phosphate cement consists of powder and liquid, which are mixed into a thick or thin consistency depending on the need. The powder comes in a variety of colors, all of which are quite bright. Hardened cement is characterized by strength and has low water absorption compared to other cements. Uncured mixed cement has a low pH until it hardens and may irritate the pulp. For deep carious cavities, additional protection of the pulp with varnish or other linear lining is required. After hardening, excess cement is easily removed in large pieces exactly along the edge of the crown.
Mix zinc phosphate cement on a glass plate. The proportion of powder and liquid depends on the purpose - liquid cement is used for cementing, thicker for gaskets and temporary fillings. Inadequate mixing degrades the properties of cement. During curing, a large amount of heat is generated, which speeds up the process. It is important to neutralize the effects of heat. Therefore, zinc phosphate cement is mixed in parts, in small portions, over the entire surface of the glass, which can be pre-cooled. To cement structures, mixing is carried out until a state is reached when, when the spatula is torn from the cement, a trace of 1-2 cm stretches behind its flat surface. To form a gasket or temporary filling, a thicker, dough-like consistency is required. At the same time, the material stops sticking to tools that have been previously coated with powder, and can be processed with a trowel and a plugger.
Hardened zinc phosphate cement, like any other cement, is almost insoluble in water, so it is better to clean tools before it hardens.
Examples include phosphate cement, Medpolymer JSC; "DeTrey Zink", Dentsply; "Adhesor", Dental Spofa; Harvard Cement, Harvard; "Phosphacap", Vivadent.
Silicate cement. It was also used back in the 19th century, mainly for filling front teeth, since at that time these were the only filling materials that allowed you to choose shades. Silicate cement served as the predecessor of the currently most common polyalkenoate or glass ionomer cements. Aluminosilicate glass in the powder, interacting with a liquid in the form of a mixture of phosphoric acids, forms a structured gel that goes through certain development phases. During the rather long (about 24 hours) maturation process, silicate cement releases free phosphoric acid, which negatively affects the living pulp. Therefore, it is not recommended to install these cements without a liner. Compared to phosphate cements, silicate cements have almost no adhesiveness to tooth tissues. Their positive property is the release of fluorine ions. The indication for use is filling cavities of classes III and V, as well as classes I and II in premolars in areas without occlusal load.
Examples include Silitsii and Med-Polymer JSC.
Silicophosphate cement. It is a mixture of silicate and phosphate cements in a ratio, usually 4:1. Due to the presence of zinc oxide in the powder, excess acid is neutralized and the adverse effect on the pulp is reduced. However, placing fillings from such cement without a spacer is allowed only for the treatment of teeth with moderate caries. Indications for use include filling cavities of classes III and V, as well as classes I and II without occlusal load.
Examples include Silidont and Med-Polymer JSC.