Glass ionomer cements as cements consist of a base component of glass and an acid component and cure through an acid-base reaction between these components. GIC powder is a finely ground fluoroaluminosilicate glass with large amounts of calcium and fluorine and small amounts of sodium and phosphates. The liquid is a solution of polycarboxylic acids (polyacrylic, polyitaconic and polymaleic). The powder is prepared by mixing the components, fusing them at a temperature of 1000-1300 degrees and after cooling, grinding to obtain a powder.
The main components of the powder:
These are compounds that determine various properties of the material:
— silicon dioxide (quartz 40%), which provides a high degree of glass transparency, slows down the setting process, lengthens the hardening time and working time, and slightly reduces the strength of hardened cement;
— aluminum oxide makes the material opaque, but increases its strength, acid resistance, reduces working time and hardening time.
— calcium fluoride reduces transparency, but increases its cariesstatic properties;
- Aluminum phosphate reduces transparency. but increases strength and mechanical stability;
- barium salts or metal compounds determine its radiopacity.
The different relationships between these components explain the development of a large number of GICs intended for use in various clinical situations.
Advantages
Glass ionomer cement mixtures used to restore the integrity of temporary and permanent teeth have the following advantages:
- high degree of adhesion to the tissues of the jaw row elements and other materials used for filling, which does not require etching with orthophosphoric acid and drying the surface;
- preventing the possibility of caries recurrence due to the long-term release of fluoride;
- biocompatibility with oral tissues , non-toxic, hypoallergenic;
- similarity in characteristics to dentin tissue , which ensures a high degree of marginal sealing of the filling and reduces the risk of chipping during operation;
- the ability to harden in an environment with high humidity , allowing the material to be used to eliminate enamel defects in the area of the tooth neck;
- low degree of shrinkage during polymerization , high strength and ductility, which provide the ability to withstand chewing load when installing orthodontic and orthopedic systems;
- acceptable aesthetic characteristics , which make it possible to fill teeth when it is impossible to use composites;
- low cost compared to composite materials.
In addition to these advantages, dentists note that glass ionomer cements are easy to apply.
This allows them to be used for the treatment of caries in children and patients who are not able to keep their mouth open for a long time, which is required by composites and compomers.
The hardening reaction of GIC occurs in three stages:
1. stage of ion formation (dissolution stage) - the acid reacts with the surface layer of glass particles to form calcium, aluminum, photor and sodium cations;
2. phase of primary gelation (hardening) - rapid cross-linking of polyacid molecules with each other, calcium and aluminum ions. At this stage, the pH of the cement begins to increase noticeably. The transformation of polyacid molecules into a gel begins;
3. stage of final hardening - strong transverse ionic bonds are formed, mainly of aluminum polyalkenate and fluorine (lasts up to 24 hours).
The trivalent nature of aluminum allows for a higher degree of cross-linking, which determines the final strength of the material. At this stage, the process of formation of silica gel on the surface of glass particles is completed.
The final structure of hardened cement is: glass particles, each of which is surrounded by silica gel, located in a matrix consisting of cross-linked polyacid molecules containing insoluble fluorine and phosphate salts.
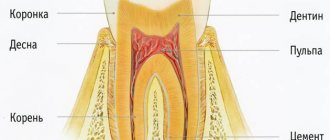
Glass ionomer cements (glass ionomers)
Glass ionomer cements (GIC) are a whole class of modern dental materials created by combining the properties of silicate and polyacrylic systems. Filling teeth using glass ionomer cements is gradually replacing zinc phosphate and zinc polycarboxylate cements from dental practice. Glass ionomer cements are usually classified according to a number of characteristics.
According to their application. For permanent fillings (aesthetic, reinforced), quick-hardening (for gaskets, fissure sealing), for filling root canals, for fixing orthopedic structures.
- By release form:
- powder-liquid (powder - finely dispersed aluminofluorosilicate glass with various additives, liquid - an aqueous solution of a copolymer of carboxylic acids with the addition of tartaric acid);
- powder (all components are in powder, which is mixed with distilled water; so-called Aqua cements);
- capsules (powder and liquid are packaged in capsules with a thin partition in the required ratio, so when mixed, glass ionomer cement with optimal properties is obtained);
- paste (in tubes or syringes); do not require mixing and harden when irradiated with a halogen lamp.
Depending on the chemical composition of the curing mechanism.
- Classic (powder-liquid). Finely dispersed aluminum fluorosilicate glass powder (particle sizes 20-50 microns). Powder components: silicon dioxide, aluminum oxide, calcium fluoride, fluorides of other metals (providing fluoride release for caries prevention), aluminum phosphate (providing strength and abrasion resistance), barium, zinc, strontium salts, etc. (providing radiopacity). The liquid is an aqueous solution of a copolymer of polycarboxylic acids (acrylic, itaconic, maleic) with the addition of a tartaric acid isomer. In the case of Aqua cements (only powder that is mixed with distilled water), polycarboxylic acids are included in the original powder in the form of crystals. In metal-containing glass ionomer cements, metal additives and alloys (silver-tin, silver-palladium) are additionally introduced into the powder composition. Hardening of classical glass ionomer cements occurs according to the type of ion exchange reaction (hence the name glass ionomer): hydrogen ions (present in an aqueous solution of polycarboxylic acids) exchange with metal ions (calcium, aluminum) of the glass, calcium and aluminum ions bind the hydroxyl groups of polycarboxylic acid chains (a matrix is formed metal polyacrylate containing unreacted glass particles). In the initial stage of hardening, calcium polyacrylic chains are formed quite quickly. This reaction ensures the cement sets and lasts for several minutes. However, the efficiency of binding by calcium ions is not high enough and in the early stages of hardening, calcium-polyacrylic chains can dissolve in water (therefore, the cement must be protected from moisture during this time). When the calcium ions have reacted, aluminum ions react and aluminum-polyacrylic chains are formed. The trivalent nature of aluminum (as opposed to the divalent nature of calcium) provides a higher degree of cross-linking and the formation of spatial structure. It is at this stage that the final cement matrix is formed. The completion of the second phase occurs in approximately 2-3 weeks (the curing process can be accelerated by the use of hybrid glass ionomers, which already gain sufficient strength within about 40 seconds at the initial stage of photopolymerization). Additionally, silica gel (strong structure) is formed on the surface of the glass particles. As a result, the final structure of hardened glass ionomer cement is glass particles surrounded by silica gel and located in a matrix of cross-linked polycarboxylic acid (metal polyacrylate) molecules.
- Hybrid glass ionomer cements (polymer modified glass ionomer cements). They have a double (chemical and light) or triple curing mechanism. The powder is finely dispersed aluminosilicate glass (as in the case of classical glass ionomer cements), sometimes with the addition of polycarboxylic acid copolymer crystals (as in the case of Aqua cements). The liquid is an aqueous solution of a copolymer of polycarboxylic acids (acrylic, itaconic, maleic), the ends of the molecules of which are modified by the addition of unsaturated methacrylate groups (as in dimethacrylates of composite filling materials). The liquid also contains tartaric acid, hydroxyethyl methacrylate and camphoroquinone (a photoinitiator). The first stage of the curing mechanism is the binding reaction of the terminal unsaturated methacrylate groups of polycarboxylic acids due to the photoinitiated formation of terminal radicals (photopolymerization). The second stage is the usual classical reaction of cross-linking of polyacid macromolecules with metal ions. Hybrid glass ionomer cements (with a dual curing mechanism) have improved physicochemical properties, but also have a significant drawback: in areas inaccessible to light from a photopolymerizing lamp, curing occurs only due to a classical chemical reaction (which affects the physicochemical characteristics of glass ionomer cements) . Glass ionomer cements with a triple curing mechanism do not have this drawback (the first two stages are like double-curing glass ionomer cements, and the third stage is catalytically initiated polymerization of the terminal methacrylate groups of polycarboxylic acids without exposure to light).
This classification is conditional, since many modified glass ionomer cements have recently appeared: with the addition of polymer resins, with specially treated fine glass particles, etc.
A very important advantage of glass ionomer cements is good chemical adhesion to tooth tissues. This is believed to occur due to the formation of chelate bonds between the hydroxyl groups of polycarboxylic acids and calcium ions of the surface hydroxyapatite (similar to the classical chemical cross-linking reaction during the hardening of glass ionomer cements), as well as due to the formation of hydrogen bonds of carboxylate groups with collagen (an organic component of dental tissues).
Other advantages of glass ionomer cements include good chemical adhesion to other filling materials (including composites), high biological compatibility with tooth tissues, thermal expansion characteristics close to tooth tissues (which protects against violation of the marginal seal of fillings), low modulus of elasticity ( which allows the use of glass ionomer cements as spacers or a base for dental restoration with composite materials).
Glass ionomer cements have bioactivity, which is associated not only with chemical adhesion to tooth structures, but also with prolonged fluoride release and the release of other ions (aluminum, calcium, strontium; they contribute to the remineralization of tooth structures during carious lesions). All other restorative materials (for example, composites) are not bioactive and serve only to restore the shape and aesthetics of the tooth. During the initial period (about 2 days) of hardening of glass ionomer cements, fluoride ions are rapidly released, which remain free within the glass ionomer matrix. The free movement (diffusion) of fluoride ions is due to the fact that they are not structurally associated with the cement matrix and are capable of migration into the oral cavity and into the dental tissue adjacent to the restoration (filling), while providing a cariesostatic and antibacterial effect. The release of fluoride ions (in smaller quantities) continues over a long period (prolonged process, more than 1 year). The diffusion of fluoride ions into dentin and enamel causes increased mineralization of hard tooth tissues, a decrease in dentin permeability, remineralization of initial carious lesions and stopping or slowing down the remaining carious process. The hard tissue under glass ionomer cement turns out to be denser and hypermineralized. In addition, glass ionomer cements are capable of adsorbing (absorbing) fluoride ions upon contact with fluoride-containing materials (toothpastes, gels, rinses), which leads to re-enrichment of the glass ionomer restoration (filling) with fluoride ions. The incoming fluoride ions are then slowly released into the oral cavity and tooth tissue adjacent to the restoration (filling). Thus, glass ionomer cement acts as a reservoir (depot) of fluoride ions. In recent years, glass ionomer cements are increasingly used to seal fissures (primarily due to the remineralizing effect on the enamel in the fissure area due to fluoride release).
Typical representatives of modern glass ionomer cements are the following.
Fuji Plus is a composite-reinforced glass ionomer cement. Used for permanent cementation of metal, metal-ceramic and metal-composite crowns and bridges, inlays and onlays made of composites, ceramics and dental alloys.
Fuji I (Fuji I) is a glass ionomer cement for permanent cementation of orthopedic crowns, bridges, inlays, onlays made of any dental alloys.
Fuji IX (Fuji 9, Fuji IX) is a classic glass ionomer restoration (filling) cement of packable viscosity (the term “packable” means maintaining the shape given to the material even before the stage of its curing, which allows the dentist to easily carry out the preliminary modeling stage). Due to its high abrasion resistance, it is used for restorations (fillings) in the area of chewing teeth and reconstruction of the crown part of the tooth.
Fuji Lining is a light-curing glass ionomer cement. It has low shrinkage during hardening, therefore it is used as an insulating gasket.
Ionosit Baseliner is a light-curing hybrid glass ionomer cement (more often referred to as compomers). A one-component material that expands slightly when curing and is therefore used as an insulating spacer to compensate for polymerization shrinkage of composites. Physical properties are approximately 3 times stronger than traditional glass ionomer cements.
TimeLine is a light-curing glass ionomer material. Used as an insulating spacer for composite fillings (restorations).
CORE MAX is a glass ionomer cement reinforced with a composite (sometimes referred to as chemically cured composites). Particularly strong cement for restoring the crown of a tooth using pins. RelyX LUTING is a chemically cured hybrid glass ionomer cement. Used for permanent cementation of orthopedic crowns, inlays made of ceramics, metals, composites, cementation of bridges, root pins. Ionoseal is a light-curing glass ionomer cement. It has high tensile strength and compression resistance. Used for insulating gaskets (has good adhesion to composite materials). Vitremer is an aesthetic hybrid glass ionomer material with a triple curing mechanism (light polymerization, chemical polymerization, classical glass ionomer reaction). Used to restore the coronal part of the tooth for prosthetics, aesthetic filling and restoration.
A shining Hollywood smile from leading specialists in therapeutic dentistry. Make an appointment!
The main positive properties of GIC:
1. Chemical adhesion to tooth tissues - occurs due to the chelate connection of the carboxyl groups of the polymeric acid molecule with calcium of the hard tooth tissues. In addition, at the final stage of hardening, a slight increase in the volume of the material occurs, which ensures a tighter edge fit. No acid etching or absolute dryness of the surface is required. The adhesion force is low and is only 8-12 MPa.
2. Chemical adhesion to most materials (cement, composites, metals, materials containing eugenol).
3. The cariesstatic and bacteriostatic effect is based on the release of fluoride during and after hardening of the cement and the formation of a layer of fluorapatites at the interface between the filling material and the tooth tissues. The effect lasts up to 6–12 months.
4. They have a “battery” effect - they are able to adsorb fluoride ions from toothpastes and, when the environment becomes acidic, release it into the surrounding tissues.
5. High biocompatibility, non-toxicity and lack of irritation to the pulp determine the use of GIC as insulating gaskets.
6. The proximity of the coefficient of thermal expansion to that of enamel and dentin prevents cracking of the material and loss of marginal seal when temperature changes in the oral cavity.
7. High compressive strength allows the use of GIC as a base for composite materials when using the “sandwich” filling technique.
8. Low elastic modulus (ability to undergo plastic deformation) allows the use of GIC when filling class 5 cavities.
9. The shrinkage of GIC is only 1.0-3.6%, which is 40% less than that of photocomposite materials.
10. Ease of use and low cost.
Advantages and disadvantages
Advantages of glass ionomers:
- Ease of use . Most GIC is introduced into the tooth cavity in 1-2 portions. The use of most of these cements does not require prior etching and bonding of enamel and dentin.
- Ability to cure in humid environments . This property allows the active use of cements for filling cervical carious cavities, wedge-shaped defects, and caries below the gum level.
- The presence of intermolecular bonds between cement and enamel or dentin . Allows you to create fillings with good marginal adhesion, and also does not require the creation of retention areas during filling.
- Adhesion to materials with clove oil . In this case, GIC can be used as isolating liners, since eugenol prevents the adhesion of composites to tissues.
- Adhesion to metals . I contacted them, GIC gives good results when used as luting material.
- "The Battery Effect" . Evidence that glass ionomers release fluoride ions into tooth tissue for at least a year from the moment of filling.
- Low polymerization shrinkage . During the hardening process, cements adsorb water from the oral fluid, which leads to a slight increase in its volume and compensates for shrinkage, which is 40% higher for light-curing composite materials.
- Inertness towards the pulp and lack of irritating effect on it . GIC promotes the formation of tertiary dentin, and also does not require etching of dentin with phosphoric acid, and has a non-aggressive composition. All this allows them to be used as a gasket when filling deep caries using the sandwich technique.
- The coefficient of thermal expansion is identical to tooth tissue . This ensures a strong marginal fit of the fillings, and also prevents chipping of the edges of enamel and dentin due to thermal expansion of the filling.
The disadvantages are mainly inherent in the “classical” GIC:
- Long maturation of the cement mass - the cement finally hardens after 24 hours, although the primary formation of cross-links occurs after 3-5 minutes.
- The first day after filling, the material is sensitive to increased humidity (ions are washed out) or its lack (the processes of ion formation in the cement mass are disrupted and polymerization takes longer).
- The structure may be damaged by vibration influences. Therefore, such fillings cannot be treated with burs immediately after placement.
- Do not etch tooth tissue (orthophosphoric acid prolongs the maturation period of the cement mass).
- In case of deep caries, the use of GIC is only possible with the use of therapeutic pads, otherwise the material will adsorb fluid from the odontoblasts in the pulp.
- Low diametric tensile strength, which makes it impossible to use cements for filling cavities on occlusal surfaces and in situations where the chewing load is distributed over the surface of the filling in different directions.
- Fast abrasion of GIC fillings compared to composite fillings.
- Lack of aesthetics.
Negative properties of “classical” GIC:
1. Duration of “maturation” of the cement mass (primary hardening is 3-6 minutes, while the final hardening is 24 hours). On the first day after filling, the material:
- sensitive to excess or lack of moisture (excess - leaching of ions; deficiency - disruption of the dissociation process and also disruption of the formation of the polymer structure). Therefore, it is necessary to coat the filling with a protective varnish.
- sensitive to mechanical stress (especially vibration when processing fillings with burs) - disruption of the formation of a chemical bond with hard tissues. Therefore, grinding and polishing of the filling must be carried out on the second visit.
— impossibility of etching the material with orthophosphoric acid (etching disrupts the maturation process of the material).
— The danger of osmotic injury to odontoblasts when applying GIC for deep caries without a therapeutic lining (since the material during this period “pulls” moisture for the polymerization process.
2. Low diametric tensile strength does not allow the use of GIC in areas of significant load, especially multidirectional.
3. GICs have low abrasion resistance, so they cannot be used in cavities with high mechanical loads.
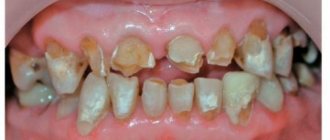
4. In terms of aesthetic properties, GICs are inferior to composite materials. Their color qualities are satisfactory and close to composites; the main problem is that GIC is close to dentin in transparency. The problem with GIC is its poor polishability.
The process of improving the JIC is currently ongoing.
Basic operating principles
“Classical” GIC and aqua cements are used using the following technology:
- treat the tooth cavity with conditioner, hold it for 20 seconds, rinse, dry;
- carefully isolate the tooth from moisture while the filling is curing (up to 6 minutes);
- Mix the powder and liquid for 30-40 seconds to obtain a glossy, homogeneous mass;
- introduce cement into the tooth cavity and model the filling, tightly condensing it with a damp cotton ball or plugger with a spherical working part;
- Coat the finished filling with varnish and treat with burs the next day.
Double-curing GIC (paste-paste type):
- etch the enamel with orthophosphoric acid, dry and isolate the tooth from moisture, apply adhesive and polymerize it according to the instructions;
- mix two pastes from the tube according to the proportion recommended by the manufacturer;
- dry the tooth cavity, carefully isolate it from moisture;
- introduce the material into the cavity, condensing it well, in a thin layer (up to 1.5 mm) and illuminate it with a photopolymerizer for the time specified in the instructions;
- repeat the previous step 2-3 times until the required filling volume is achieved;
- treat the filling with burs and rubber bands.
Triple Cure GIC:
- etch the tooth tissue with orthophosphoric acid, rinse it off and dry the cavity;
- isolate the working area from oral fluid;
- apply the adhesive system according to the instructions using a microbrush;
- blow out the cavity, illuminate the bond;
- mix the powder and liquid, add the material into the cavity in one portion, condensing it tightly;
- grind and polish the filling.
One-component cements modified with composite resins:
- etch tissues with orthophosphoric acid for 40 seconds on enamel and 20 on dentin;
- wash off the etching gel, isolate the tooth from oral fluid, dry the cavity;
- apply the bond using a microbrush, blow out the tooth cavity, illuminate it with a photopolymerizer;
- apply the material according to the instructions for it, rubbing tightly against the walls of the tooth;
- expose each layer for the time specified in the instructions;
- grind and polish the filling.
Traditional GICs are divided into three groups depending on their clinical application:
Type 1 - fixing or luting are used for fixing inlays and onlays, crowns and bridges, and orthodontic appliances. An important requirement of these GICs is the ability to obtain a thin film with a thickness of 11-13 microns between the tooth surface and the crown. Distinctive features of this group are reduced particle sizes, powder-liquid ratio 1.5:1, long working time (Aqua-cem, Dentsply; Fuji 1, Ji Si; Ketak-bond, ESPE.)
Type 2 – restorative to restore defects in teeth. They have high strength and lower solubility compared to other groups, due to modification of the glass composition and a high powder-liquid ratio of 3:1. Hardening lasts on average 5-7 minutes.
1 subtype – “aesthetic”. By increasing silicon oxide, aesthetic properties are improved, but strength is reduced, hardening time is extended, and sensitivity to moisture increases. Indications: cervical defects of anterior teeth (class 5 according to Black, enamel erosion, wedge-shaped defects); small cavities class 1; class 3 cavities; caries of the roots of the frontal teeth.
Subtype 2 – “hardened” GIC. Special fibers and metal additives are introduced into the powder. These materials are inferior in aesthetic properties to materials of subtype 1, but have greater strength and a higher rate of hardening with early resistance to moisture. These cements can be etched with acid if the layer thickness is at least 1 mm (Fuji 2 and 9, GSI; HemFlex, Dentsply; Ionofil, Voko). Indications – caries of primary teeth (cavities of classes 1 and 2); caries of the roots of chewing teeth; small cavities class 1; ART technique; temporary fillings up to 1 year; sealing fisser; for the “sandwich technique”.
Type 3 - lining as a lining for amalgam and composites. The requirements for them are short working time and hardening time, radiopacity, powder-liquid ratio from 1.5:1 to 4:1 depending on the required strength. Hardening time is 4-5 minutes. GIC of this group is not etched, but if the manufacturer allows this possibility, then the layer thickness should not be less than 1 mm. (Aqua Cenite, Ionobond; Aqua Ionobond, Voko; Base Line, dentsply).
Classification
There are several production forms of glass ionomer cements:
Powder-liquid. Traditional compositions. Contains finely dispersed aluminofluorosilicate glass with additional components and liquids for mixing. Divided into:
- fixing: used for fastening inlays, onlays, crowns, bridges, orthodontic structures;
- restorative: used to restore defects, including those on the front teeth;
- lining: serve as spacers between metal, composite fillings and dental tissues.
The powder is aquacement. The powder contains all the necessary components and is mixed with water.
Capsules. Liquid and powder in the required quantity are packaged in capsules. This eliminates dosage errors when mixing.
Pastes in tubes. They do not require mixing and contain light-curing components.
Restoration GICs are divided into two subtypes: aesthetic and hardened.
Additional Information! Restoration GICs are divided into two subtypes: aesthetic and hardened. The former contain more silicon oxide, making them suitable for the restoration of incisors and canines. However, such compositions are less durable. The hardened ones contain metal additives. Aesthetic qualities are reduced, but strength increases significantly. They are used on molars and for the treatment of baby teeth.
Chemically cured aqua cements.
Presented in the form of a powder that contains fluoroaluminosilicate glass with the addition of polyacid dried at a low temperature and turned into powder. Mix with distilled water. The use of these cements allows us to ensure an optimal glass-acid ratio. These materials have the same disadvantages as “classical” GICs.
One-component light-curing GICs have a polymer matrix that hardens under the influence of light and a glass ionomer filler, but during curing only the photopolymerization reaction of the polymer occurs; no glass ionomer reaction occurs in them.
A new direction was the inclusion of light-curing polymer resin in the GIC composition (hybrid GIC, rubber polymers, GIC modified by polymers). The materials are presented in the form of powder and liquid.
They have two curing mechanisms:
1. Under the influence of light from a photopolymerizer, a “fast” polymerization reaction of the polymer matrix occurs, which creates a dense frame at the initial stage of hardening.
2. Immediately after mixing the powder and liquid, the typical GIC reaction begins, lasting up to 24 hours.
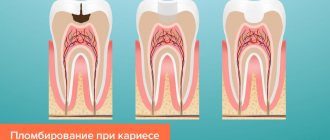
Mode of application
Tooth filling using glass ionomer cement is carried out as follows:
- Preparing the cavity. The element of the jaw row that requires restoration is protected from moisture, saliva and other foreign particles using a rubber dam.
- The cavity is prepared , tissues affected by caries are removed, and the walls of the hole are cleaned and treated.
- The cleaned cavity is covered with polyacrylic acid for 10 - 15 seconds , after which it is washed and thoroughly dried.
- Mixing the material. Depending on the form of release of the cement mass, a specialist performs actions to mix its components - powdery elements and a liquid substance.
- Adding cement. Using special plastic spoons or guns, glass ionomer material is placed into the treated cavity. If necessary, special matrices are used to create the contour of the tooth surface.
- To avoid dehydration of the filling after removal of the matrix tape, the cement material is coated with a waterproof compound.
- Finishing the filling. After the filling has completely hardened, which occurs 24 hours after its placement, excess material is removed and the surface is polished.
The lifespan of a filling made from glass ionomer cement depends both on the quality of the starting material and on the actions of the dentist during its placement.
The video shows an example of the use of a hybrid glass ionomer material.
Positive properties of dual-curing hybrid GIC:
1. less sensitive to moisture and dehydration;
2. have improved strength characteristics compared to “traditional” ones;
3. harden without the formation of microcracks;
4. have increased adhesion force to tooth tissues.
Negative properties of dual-curing hybrid GIC:
1. the polymer matrix hardens only under the influence of light from a photopolymerizer;
2. strength characteristics and color range are worse than those of FKM. Triple-cure hybrid GIC
Work to improve glass ionomers led to the creation of the hybrid cement “Vitrimer” (ZM E5PE). This is the only glass ionomer cement that uses triple curing technology:
1) light curing of the polymer matrix occurs directly during light irradiation. This makes it possible to achieve high strength already in the process of applying the filling, ensures ease of use, and reduces the possibility of contamination;
2) chemical curing of the polymer matrix is ensured by the powder containing microcapsules with a proprietary catalytic system. When the powder is mixed with liquid, the capsules are destroyed and the catalyst is activated. The presence of a mechanism for chemical curing of the polymer matrix of the material ensures guaranteed complete curing of all areas of the filling, even without light irradiation. Thus, there is no need for layer-by-layer application of material. Applying a filling, even a large one, using only one portion of material allows you to obtain a uniform structure and significantly saves time;
3) the “classical” glass ionomer curing reaction, characteristic of all glass ionomers, lasts for 24 hours and occurs inside a durable polymer “frame”. The glass ionomer reaction provides Vitrimer with chemical adhesion to the hard tissues of the tooth, biocompatibility, prolonged release of fluoride, and, consequently, high quality restoration and a reduced likelihood of developing “recurrent” caries.
Indications for use of the hybrid glass ionomer triple-curing system “Vitrimer” (ZM E5PE):
1. Aesthetic filling of carious cavities of classes III and VI in adults.
2. Filling of non-carious tooth defects: erosion, wedge-shaped defects, etc.
3. Filling cavities of all classes in baby teeth.
4. Dental filling in gerontostomatology.
5. Temporary restoration of broken teeth.
6. Restoration of a destroyed tooth crown with the creation of a stump for the crown (blue tint).
7. Basic gasket when filling a tooth using the “sandwich” method.
8. In case of unsatisfactory oral hygiene, high incidence of “recurrent” caries, filling of tooth root defects.
Application
The main area of application of glass ionomer cement is the treatment of superficial and medium caries on chewing surfaces. In addition, it is used for:
- fissure sealing;
- gaskets when installing amalgams and composites;
- canal filling;
- fixation of orthopedic and orthodontic structures, maxillofacial orthopedic bandages;
- therapy of cervical, secondary, progressive caries.
After 2–3 weeks, the glass ionomer filling will darken slightly.
Important! GIC cannot be used for filling large cavities with deep caries and chronic mouth breathing. In the first case, this will lead to inflammation of the pulp, in the second - to possible drying out of the material.
Manufacturers' offers
There are a large number of glass polyalkenate cements on the dental market.
Their diversity is explained by differences in composition, hardening technology and purpose.
Vitremer
The American-made composition Vitremer is an innovative material with a three-phase curing mechanism, which involves light, chemical and glass ionomer curing.
This mass is easy to apply because it does not require layer-by-layer application, and also has high strength and aesthetic characteristics.
The advantages include the following points:
- high adhesion to dentin;
- resistance to moisture in the oral cavity even without the use of a rubber dam;
- long-term release of fluoride;
- high radiopacity.
Vitremer can be used both for the restoration of damaged teeth and for the purpose of fixing orthodontic and orthopedic structures.
Fuji IILC
Chemically cured glass ionomer cement Fuji IILC is most often used for filling cavities of Black class 3 and 5, restoring baby teeth, and fixing pins.
Dentists note the following advantages of the material:
- reliability of use at high humidity;
- high degree of marginal fit;
- long-term release of fluoride;
- good adhesion to dental tissues;
- high radiopacity.
The product is available in the form of powder and liquid, the mixing time of which does not exceed 30 seconds.
SHALON-PHIL
German filling material belongs to the category of polymaleic glass ionomer cements. Its connection with dentin and enamel occurs on a chemical basis.
This ensures a strong sealing of the carious cavity and prevents the recurrence of caries in it.
Manufacturers of the mass recommend using it to restore the integrity of anterior teeth, restore wedge-shaped defects and enamel damage, and eliminate carious cavities of classes 3 and 5 according to Black.
The recommended mixing time for the material is 1 minute, the application process is 2 minutes, and primary curing occurs 7 minutes after preparing the cement mass.
KETAC-MOLAR
Glass ionomer radiopaque material KETAK-MOLAR is used for restoration of primary and permanent chewing teeth, restoration of tooth stump, splinting, and temporary filling.
Due to its high adhesion to dentin, the composition ensures a good marginal fit of the filling, eliminating the possibility of chipping its edges when creating a chewing load.
Among the advantages are also noted its ability to release fluoride ions, radiopacity and the ability to use without an insulating pad . The material is available in several color shades, which allows you to choose a mass that best matches the natural tone of the enamel.
KETAK-MOLAR is available in the form of powder, packaged in 15-gram bottles, and liquid for dilution. The kit also includes a special measuring spoon.