To remove the pulp, it is first killed. For this purpose, arsenic, or rather pastes based on it, has been used in dentistry for many years. And although during this time many different anesthesia, effective and modern, have appeared, it is still used to this day, although not as often as before. Although this is quite a difficult matter. If you do not accurately calculate the dosage of the drug or the time it should remain in the tooth, there may be unpleasant side effects for the tooth, and even for the patient’s entire body.
What is the purpose of adding arsenic?
Arsenic is known as a toxic substance, its degree of danger is very high. But based on it, necrotizing pastes have been developed, which have been used in dentistry for a long time.
To this day, pastes containing arsenic are used to necrotize the pulp of a diseased tooth.
They are still used in situations where it is impossible to use modern drugs for pain relief. Most often due to the patient's individual intolerance to anesthesia or when there is an urgent need to kill the nerve during treatment.
The toxic chemical has a destructive effect on blood vessels and nerve endings, which makes the pulp dead. The paste combines arsenic with an anesthetic, so the patient does not feel pain during nerve necrosis.
Providing first aid in case of poisoning
If a person is intoxicated with arsenic compounds, you need to:
- call an ambulance or emergency help;
- remove the person from the zone of action of the poison;
- to reduce the effect of arsenic on the skin, the poison from the surface of the skin should be washed off with soap and water, and the eyes should be washed with a 2% soda solution;
- do a gastric lavage with two liters of water and four teaspoons of table salt; give activated carbon (one tablet per kilogram of weight);
- If breathing and cardiac activity stop, carry out resuscitation measures.
To completely remove the toxin from the body, you need specific medical care, which can only be provided in a hospital.
To avoid getting poisoned by arsenic and its compounds, you must strictly adhere to the rules for working with toxic substances and storage rules. It is prohibited to store poisons in the kitchen or food warehouses so that they do not accidentally get into food.
Treatment procedure
The procedure for removing the nerve is a two-step process.
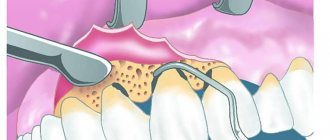
At the first stage, the dentist opens the tooth cavity affected by caries, removes dead tissue that has already been affected by the disease, and thoroughly cleans the inside of the tooth with a bur so that there are no infected areas left. A preparation containing arsenic is placed into the cavity prepared in this way. The cavity is then closed with a temporary filling. The patient goes home and soon returns to complete the tooth treatment, after a certain period of time, which is set by the doctor.
The second stage is that the dentist removes the temporary filling, removes the paste with arsenic, and cleans the inner surface again, determining by the remnants of the dye that is in the paste that it has not yet been completely removed. Before removing the nerve, the dentist must ensure that there are no traces of arsenic inside the tooth.
Depulpation - removal of the dead nerve takes place without any sensation of pain on the part of the patient.
At the end of the treatment procedures, the patient may be sent for an X-ray of the tooth to make sure that everything was done correctly and the treatment was effective.
What does arsenic look like in examples?
The patient will not be able to look at the arsenic that is in his tooth; it is sealed with a temporary filling. If it suddenly falls out, you need to be sure that there is no paste with arsenic in your mouth. You should rinse your mouth with a soda solution with iodine, plug the hole in the tooth with a ball of cotton wool or bandage, and consult a doctor immediately or as quickly as possible.
If the top of the tooth is bluish, it means arsenic has come into contact with it.
It is easy to see that the paste has entered your mouth by the blue color that is added to it. If the top of the tooth is bluish, it means arsenic has come into contact with it. It needs to be removed urgently.
It is possible that some of the paste was swallowed. There is no need to be afraid, the dose is not lethal. But just in case, to neutralize its negative effects, you need to drink 200-250 ml of milk.
Symptoms
The characteristic symptoms of arsenic poisoning depend on the dose of the substance received.
The lethal dose for a person due to arsenic poisoning, if arsenic trioxide was ingested, is between 50 and 340 mg. Its value directly depends on the state of health and weight of the person, as well as what type of toxic substance was used.
For arsenic hydrogen, the lethal indicators are as follows:
- inhalation of gas for 15 minutes with a concentration of 0.6 mg/l;
- 5 min – 1.3 mg/l;
- several breaths – 2-4 mg/l;
- instantly – 5 mg/l.
Signs of poisoning depend on the type of lesion:
- Acute form - there is a metallic taste in the mouth, accompanied by a burning sensation in the throat and spasms of the larynx. The skin becomes bluish, and the sclera of the eyes and palms turn yellow. Blood pressure drops and severe attacks of dizziness occur. Acute renal and liver failure develops. The stomach hurts severely and uncontrollable diarrhea occurs, which quickly removes fluid from the body, resulting in dehydration. In severe cases, possible: spasm or pulmonary edema, paralysis, loss of consciousness and coma.
- Subacute form – severe irritation of the eyes and mucous membranes, leading to watery eyes and a “runny nose.” Sneezing, coughing and chest tightness. Nausea and vomiting are possible, with a metallic aftertaste in the mouth. I have a particularly severe headache.
- The chronic form is anemic conditions, general malaise and rapid physical fatigue. Weakness of the limbs, loss of peripheral sensitivity, numbness of skin areas and “pins and needles” occur. Sustained rosacea, telangiectasia and spider veins develop throughout the body. Dangerous consequences are possible - the development of encephalopathy and toxic hepatitis. Due to its high carcinogenicity, arsenic can be an impetus for the development of cancer.
A typical sign of chronic arsenic poisoning is white stripes on the nails.
In men who work for a long time in hazardous work, arsenic poisoning causes symptoms and the following changes:
- hyperkeratosis - excessive growth of the surface layers of the skin;
- dryness, peeling and peeling of the skin on all parts of the body;
- increased red pigmentation in the temples, eyelids, neck, armpits, nipples and scrotum;
- Transverse white stripes appear on the nails.
How long to keep arsenic?
The duration depends on the complexity of the dental problem being solved. The minimum period of nerve killing is 48 hours. If we are talking about a tooth with one root, then the nerve can be killed by arsenic in one day. The maximum that is allowed for using such pastes is 3 days.
How long can a child keep arsenic?
When treating baby teeth, arsenic is applied for a shorter period - from 16 to 24 hours. If you leave arsenic paste inside the tooth, then negative consequences and complications are likely:
- Dentin darkens
- The body becomes intoxicated
- Periodontitis due to exposure to paste (medicinal)
- Pulp swelling
- Dieback of the periosteum
Notes[ | ]
- ↑ 1234
Sabina C. Grund, Kunibert Hanusch, Hans Uwe Wolf “Arsenic and Arsenic Compounds” in Ullmann's Encyclopedia of Industrial Chemistry, VCH-Wiley, 2008, Weinheim. (English) - Giant Mine - Northwest Territories Region - Indian and Northern Affairs Canada (unspecified)
(inaccessible link). Retrieved August 28, 2007. Archived April 2, 2012. (English) - Holleman, A.F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- Marcel Gielen, Edward RT Tiekink.
Metallotherapeutic Drugs and Metal-Based Diagnostic Agents. - Wiley, 2005. - P. 298. - Steven L. Soignet et al.
United States Multicenter Study of Arsenic Trioxide in Relapsed Acute Promyelocytic Leukemia (English) // Journal of Clinical Oncology (English) Russian. : journal. - 2001. - Vol. 19, no. 18. - P. 3852—3860. - Antman, KH
Introduction: The history of arsenic trioxide in cancer therapy (English) // Oncologist: journal. - 2001. - Vol. 6(Suppl. 2), no. 1-2. - P. 2006. - Jun Zhu, Zhu Chen, Valérie Lallemand-Breitenbach, Hugues de Thé “How Acute Promyelocytic Leukaemia Revived Arsenic,” Nature Reviews Cancer 2002, volume 2, 1-9.
- Bobé Pierre, Bonardelle Danielle, Benihoud Karim, Opolon Paule, Chelbi-Alix Mounira.
Arsenic trioxide: A promising novel therapeutic agent for lymphoproliferative and autoimmune syndromes in MRL/lpr mice (English) // Blood (English) Russian. : journal. - American Society of Hematology (English) Russian, 2006. - Vol. 108, no. 13. - P. 3967—3975.. - Lu J., Chew EH, Holmgren A.
Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide (English) // Proceedings of the National Academy of Sciences of the United States of America: journal. - 2007. - Vol. 104, no. 30. - P. 12288—12293. - doi:10.1073/pnas.0701549104. - PMID 17640917. - Stanton v Benzler 9716830 (unspecified)
. US 9th Circuit Court of Appeals (June 17, 1998). — “(...) sad by a jury of first degree murder for poisoning her ex-husband. Her ex-husband's body was found with traces of arsenic trioxide in it." Retrieved June 9, 2008. Archived April 2, 2012. - ↑ 1 2 Emsley, John.
Arsenic // The Elements of Murder: A History of Poison (English). - Oxford University Press, 2006. - P. 93 -197. — ISBN 9780192806000. - Madame Bovary by Flaubert
- Arsenic Eaters - New York Times July 26, 1885
- Richard M. Allesch. Arsenik. Seine Geschichte in Österreich. 54.Band. Klagenfurt: Kleinmayr 1959.
- G. Przygoda, J. Feldmann, W. R. Cullen.
The arsenic eaters of Styria: a different picture of people who were chronically exposed to arsenic (English) // Applied Organometallic Chemistry (English) Russian. : journal. - 2001. - Vol. 15, no. 6. - P. 457-462. - doi:10.1002/aoc.126.
The dangers of using arsenic in dental treatment
Now dentists try to use arsenic as little as possible.
This is due to the seriousness of side effects and the growing number of contraindications:
- Allergic reactions of patients to paste components
- Childhood
- Tooth canals are severely crooked
- There is a possibility of increased intraocular pressure
- Presence of kidney disease
- The integrity of the root may be affected
The use of arsenic during pregnancy is undesirable
They try not to use arsenic-based pastes for depulping teeth in pregnant women due to the unstudied effect of such drugs on the unborn child and its intrauterine development.
The danger also lies in a possible overdose and exceeding the period of arsenic remaining inside the tooth. The result may be the death of healthy tissue around.
A temporary filling falls out quite often, so toxic components, although in small quantities, enter the oral cavity or stomach. Therefore, there is a high probability of arsenic poisoning of a dental patient.
If you are personally intolerant to the components of the paste, problems with the liver and gastrointestinal tract may occur after its use.
In dental treatment in children, arsenic is rarely used, with great caution, and only if the tooth roots are fully formed.
Arsenic in nature.
There is little arsenic in the earth's crust - about 5·10–4% (that is, 5 g per ton), approximately the same as germanium, tin, molybdenum, tungsten or bromine. Arsenic is often found in minerals together with iron, copper, cobalt, and nickel.
The composition of minerals formed by arsenic (and about 200 of them are known) reflects the “semi-metallic” properties of this element, which can be in both positive and negative oxidation states and combine with many elements; in the first case, arsenic can play the role of a metal (for example, in sulfides), in the second - a non-metal (for example, in arsenides). The complex composition of a number of arsenic minerals reflects its ability, on the one hand, to partially replace sulfur and antimony atoms in the crystal lattice (the ionic radii of S–2, Sb–3 and As–3 are close and are 0.182, 0.208 and 0.191 nm, respectively), on the other – metal atoms. In the first case, arsenic atoms have a rather negative oxidation state, in the second - a positive one.
The electronegativity of arsenic (2.0) is small, but higher than that of antimony (1.9) and most metals, therefore the –3 oxidation state is observed for arsenic only in metal arsenides, as well as in stibarsen SbAs and intergrowths of this mineral with pure crystals antimony or arsenic (mineral allemontite). Many arsenic compounds with metals, judging by their composition, are intermetallic compounds rather than arsenides; some of them have variable arsenic content. Arsenides may simultaneously contain several metals, the atoms of which, at close ion radii, replace each other in the crystal lattice in arbitrary ratios; in such cases, in the mineral formula, the symbols of the elements are listed separated by commas. All arsenides have a metallic luster; they are opaque, heavy minerals, and their hardness is low.
Examples of natural arsenides (about 25 of them are known) are the minerals löllingite FeAs2 (an analogue of pyrite FeS2), skutterudite CoAs2–3 and nickelskutterudite NiAs2–3, nickel (red nickel pyrite) NiAs, rammelsbergite (white nickel pyrite) NiAs2, safflorite (Space cobalt ) CoAs2 and clinosafflorite (Co,Fe,Ni)As2, langisite (Co,Ni)As, sperrylite PtAs2, maucherite Ni11As8, oregonite Ni2FeAs2, algodonite Cu6As. Due to their high density (more than 7 g/cm3), geologists classify many of them as “super-heavy” minerals.
The most common arsenic mineral is arsenopyrite (arsenic pyrite). FeAsS can be considered as a product of the replacement of sulfur in FeS2 pyrite with arsenic atoms (ordinary pyrite also always contains some arsenic). Such compounds are called sulfosalts. Similarly, the minerals cobaltine (cobalt luster) CoAsS, glaucodote (Co,Fe)AsS, gersdorfite (nickel luster) NiAsS, enargite and luzonite of the same composition, but different structures Cu3AsS4, proustite Ag3AsS3 - an important silver ore, which is sometimes called “ruby silver”, were formed. Because of its bright red color, it is often found in the upper layers of silver veins, where magnificent large crystals of this mineral are found. Sulfosalts may also contain noble metals of the platinum group; These are the minerals osarsite (Os,Ru)AsS, ruarsite RuAsS, irarsite (Ir,Ru,Rh,Pt)AsS, platarsite (Pt,Rh,Ru)AsS, hollingworthite (Rd,Pt,Pd)AsS. Sometimes the role of sulfur atoms in such double arsenides is played by antimony atoms, for example, in seinajokite (Fe,Ni)(Sb,As)2, arsenopalladinite Pd8(As,Sb)3, arsenepolybasite (Ag,Cu)16(Ar,Sb)2S11 .
The structure of minerals is interesting, in which arsenic is present simultaneously with sulfur, but plays rather the role of a metal, grouping together with other metals. These are the minerals arsenosulvanite Cu3(As,V)S4, arsenogauchekornite Ni9BiAsS8, freibergite (Ag,Cu,Fe)12(Sb,As)4S13, tennantite (Cu,Fe)12As4S13, argentotennantite (Ag,Cu)10(Zn,Fe) 2(As,Sb)4S13, goldfieldite Cu12(Te,Sb,As)4S13, gyrodite (Cu,Zn,Ag)12(As,Sb)4(Se,S)13. You can imagine what a complex structure the crystal lattice of all these minerals has.
Arsenic has a clearly positive oxidation state in natural sulfides - yellow orpiment As2S3, orange-yellow dimorphite As4S3, orange-red realgar As4S4, carmine-red getchellite AsSbS3, as well as in colorless oxide As2O3, which occurs in the form of arsenolite and claudetite minerals with different crystalline structure (they are formed as a result of weathering of other arsenic minerals). Typically these minerals are found in the form of small inclusions. But in the 30s of the 20th century. In the southern part of the Verkhoyansk Range, huge crystals of orpiment measuring up to 60 cm in size and weighing up to 30 kg were found.
In natural salts of arsenic acid H3AsO4 - arsenates (about 90 of them are known), the oxidation state of arsenic is +5; examples include bright pink erythrin (cobalt color) Co3(AsO4)2 8H2O, green annabergite Ni3(AsO4)2 8H2O, scorodite FeIIIAsO4 2H2O and simplesite FeII3(AsO4)2 8H2O, brown-red gasparite (Ce, La,Nd)ArO4, colorless goernesite Mg3(AsO4)2 8H2O, rooseveltite BiAsO4 and kettigite Zn3(AsO4)2 8H2O, as well as many basic salts, for example, olivenite Cu2AsO4(OH), arsenobismite Bi2(AsO4)(OH) 3. But natural arsenites, derivatives of arsenic acid H3AsO3, are very rare.
In central Sweden there are the famous Langbanov iron-manganese quarries, in which more than 50 samples of arsenate minerals were found and described. Some of them are not found anywhere else. They were once formed as a result of the reaction of arsenic acid H3AsO4 with pyrocroite Mn(OH)2 at not very high temperatures. Typically, arsenates are oxidation products of sulfide ores. They, as a rule, have no industrial use, but some of them are very beautiful and adorn mineralogical collections.
In the names of numerous arsenic minerals one can find place names (Lölling in Austria, Freiberg in Saxony, Seinäjoki in Finland, Skutterud in Norway, Allemon in France, the Canadian Langis mine and Getchell mine in Nevada, Oregon in the USA, etc.), the names of geologists, chemists, politicians, etc. (German chemist Karl Rammelsberg, Munich mineral trader William Maucher, mine owner Johann von Gersdorff, French chemist F. Claudet, English chemists John Proust and Smithson Tennant, Canadian chemist F. L. Sperry, US President Roosevelt, etc.), names of plants (thus, the name of the mineral safflorite comes from saffron), the initial letters of the names of the elements - arsenic, osmium, ruthenium, iridium, palladium, platinum, Greek roots (“erythros” - red, “enargon” - visible, “lithos” - stone) and etc. and so on.
An interesting ancient name for the mineral nickel (NiAs) is kupfernickel. Medieval German miners called Nickel the evil mountain spirit, and “kupfernickel” (Kupfernickel, from German Kupfer - copper) - “damn copper”, “fake copper”. The copper-red crystals of this ore looked very much like copper ore; It was used in glass making to color glass green. But no one was able to get copper from it. This ore was studied by the Swedish mineralogist Axel Kronstedt in 1751 and isolated a new metal from it, calling it nickel.
Since arsenic is chemically quite inert, it is also found in its native state - in the form of fused needles or cubes. Such arsenic usually contains from 2 to 16% impurities - most often these are Sb, Bi, Ag, Fe, Ni, Co. It is easy to grind into powder. In Russia, geologists found native arsenic in Transbaikalia, in the Amur region, and it is also found in other countries.
Arsenic is unique in that it is found everywhere - in minerals, rocks, soil, water, plants and animals; it is not without reason that it is called “ubiquitous”. The distribution of arsenic over different regions of the globe was largely determined during the formation of the lithosphere by the volatility of its compounds at high temperatures, as well as by the processes of sorption and desorption in soils and sedimentary rocks. Arsenic migrates easily, which is facilitated by the fairly high solubility of some of its compounds in water. In humid climates, arsenic is washed out of the soil and carried away by groundwater and then by rivers. The average arsenic content in rivers is 3 µg/l, in surface waters – about 10 µg/l, in sea and ocean waters – only about 1 µg/l. This is explained by the relatively rapid precipitation of its compounds from water with accumulation in bottom sediments, for example, in ferromanganese nodules.
In soils, the arsenic content is usually from 0.1 to 40 mg/kg. But in areas where arsenic ores occur, as well as in volcanic areas, the soil can contain a lot of arsenic - up to 8 g/kg, as in some areas of Switzerland and New Zealand. In such places, vegetation dies and animals get sick. This is typical for steppes and deserts, where arsenic is not washed out of the soil. Clay rocks are also enriched compared to the average content - they contain four times more arsenic than the average. In our country, the maximum permissible concentration of arsenic in soil is 2 mg/kg.
Arsenic can be carried out of the soil not only by water, but also by wind. But to do this, it must first turn into volatile organoarsenic compounds. This transformation occurs as a result of the so-called biomethylation - the addition of a methyl group to form a C–As bond; this enzymatic process (it is well known for mercury compounds) occurs with the participation of the coenzyme methylcobalamin, a methylated derivative of vitamin B12 (it is also found in the human body). Biomethylation of arsenic occurs in both fresh and sea water and leads to the formation of organoarsenic compounds - methylarsonic acid CH3AsO(OH)2, dimethylarsine (dimethylarsenic, or cacodylic) acid (CH3)2As(O)OH, trimethylarsine (CH3)3As and its oxide (CH3)3As = O, which also occur in nature. Using 14C-labeled methylcobalamin and 74As-labeled sodium hydroarsenate Na2HAsO4, it was shown that one of the methanobacterial strains reduces and methylates this salt to volatile dimethylarsine. As a result, the air in rural areas contains an average of 0.001 - 0.01 μg/m3 of arsenic, in cities where there is no specific pollution - up to 0.03 μg/m3, and near sources of pollution (non-ferrous metal smelting plants, power plants operating on coal with a high arsenic content, etc.) the concentration of arsenic in the air can exceed 1 μg/m3. The intensity of arsenic deposition in areas where industrial centers are located is 40 kg/km2 per year.
The formation of volatile arsenic compounds (trimethylarsine, for example, boils at only 51°C) caused in the 19th century. numerous poisonings, since arsenic was contained in plaster and even green wallpaper paint. n were previously used as paint
H2O and Parisian or Schweyfurt greens Cu4(AsO2)6(CH3COO)2. In conditions of high humidity and the appearance of mold, volatile organoarsenic derivatives are formed from such paint. It is believed that this process could be the reason for the slow poisoning of Napoleon in the last years of his life (as is known, arsenic was found in Napoleon's hair a century and a half after his death).
Arsenic is found in noticeable quantities in some mineral waters. Russian standards establish that arsenic in medicinal table mineral waters should not exceed 700 µg/l. In Jermuk
it may be several times larger. Drinking one or two glasses of “arsenic” mineral water will not cause harm to a person: to be fatally poisoned, you need to drink three hundred liters at once... But it is clear that such water cannot be drunk constantly instead of ordinary water.
Chemists have found that arsenic in natural waters can be found in different forms, which is significant from the point of view of its analysis, migration methods, as well as the different toxicity of these compounds; Thus, compounds of trivalent arsenic are 25–60 times more toxic than pentavalent arsenic. As(III) compounds in water are usually present in the form of weak arsenic acid H3AsO3 ( pK
a = 9.22), and the As(V) compound - in the form of a much stronger arsenic acid H3AsO4 (
pK
a = 2.20) and its deprotonated anions H2AsO4– and HAsO42–.
Living matter contains an average of 6·10–6% arsenic, that is, 6 µg/kg. Some seaweeds can concentrate arsenic to such an extent that they become dangerous to humans. Moreover, these algae can grow and reproduce in pure solutions of arsenous acid. Such algae are used in some Asian countries as a remedy against rats. Even in the clear waters of the Norwegian fjords, algae can contain up to 0.1 g/kg of arsenic. In humans, arsenic is found in brain tissue and muscles, and it accumulates in hair and nails.
More details about the pros and cons
How to remove paste with arsenic from a tooth yourself?
Dentists have a negative attitude towards patients removing the drug themselves, and it is difficult to do it completely at home. But there are times when it needs to be done. For example, when the clinic has a day off or a holiday, and the treatment was emergency, and it’s time to remove the composition. Or the filling fell out prematurely. If you need to remove arsenic, for example, on a hike, away from the city. How it's done? If the temporary filling is in place, it can be pulled out using the needle of a disposable syringe or a sewing needle. The material of such a filling is not very durable. If the filling is no longer present, you need to remove the arsenic composition. First, the needle or tweezers are disinfected in alcohol and the paste is removed. After this, the mouth is thoroughly rinsed with a solution of bicarbonate (soda) with hydrogen peroxide or iodine. The hole in the tooth must be closed with a cotton or gauze swab. After this, you need to contact your dentist as soon as possible. Otherwise, the likelihood of severe inflammation and other dental complications increases.
Possible causes of poisoning
Is it possible to get poisoned by arsenic today? Of course yes, because none of the workers is insured against accidents at work, and when using arsenic-based poisons in everyday life, it may accidentally enter the body. Sometimes intentional cases of poisoning are recorded - suicide or murder. All of these episodes are classified as acute poisoning.
Arsenic poisoning can also occur through occupational exposure to small doses, as well as through prolonged consumption of contaminated water or taking medications. Such poisonings are classified as chronic.
A special, subacute category of poisoning includes cases of a person coming into contact with adamsite, which is used by police in some countries to disperse mass demonstrations. In poisons classified as chemical warfare agents, adamsite occupies a position among sternites - compounds that irritate the upper respiratory tract.
Another common cause of arsenic poisoning is the collection of mushrooms in places where chemical weapons are destroyed or unscrupulous disposal of waste containing arsenic. In the fruiting bodies of mushrooms growing in such areas, the concentration of arsenic exceeds the permissible limit by 1,000 times, but they taste and smell no different from the same mushrooms growing in neighboring “clean” areas. Moreover, scientists have come to the conclusion that mycelia prefer soils rich in arsenic, so eating mushrooms purchased second-hand without appropriate laboratory analysis is quite reckless.
We should not forget that acute, subacute or chronic arsenic poisoning can also be caused by improper washing of vegetables or fruits, since arsenic-based preparations are actively used to control rodents in storage facilities.
Should pain occur during such treatment and how to get rid of it?
How long does it take for a tooth to be bothered after placing arsenic under a temporary filling?
After removing the temporary filling with arsenic, further treatment is painless
If the dentist performed all the manipulations correctly, then there should be no pain at all. Because the paste contains a component with an anesthetic effect - this is the first thing. Secondly, nerve necrosis under the influence of arsenic means that the tooth loses sensitivity completely.